Mark Braiman

Mark Braiman
Professor Emeritus
CONTACT
Chemistry
3-006 Center for Science and Technology
Email: mbraiman@syr.edu
PROGRAM AFFILIATIONS
Biotechnology
Energy and Its Impacts [ILM]
Solar powered photoreduction of CO2; microbial rhodopsins and G-protein-coupled receptors; time-resolved vibrational spectroscopy
- B.S., 1977, Harvard University
- Ph.D., 1983, University of California at Berkeley
- Postdoctoral Fellow, 1983-86, MIT
- Postdoctoral Fellow, 1986-1988, Boston University
- National Science Foundation Predoctoral Fellowship, 1977-1980
- Helen Hay Whitney Postdoctoral Fellowship, 1983-86
- Lucille P. Markey Scholar Award, 1986-1992
- CHE 106: General Chemistry Lecture
- CHE 335: Chemical and Biochemical Analysis
- CHE 346: Physical Chemistry
- CHE 467: Introduction to Physical Chemistry Research Laboratory
- CHE 474/674: Structural and Physical Biochemistry
- CHE/BCM 477/677: Biochemistry Structural Laboratory
Interactions of molecules with light are a unifying theme for my group's research. A long-standing focus is on membrane proteins that bind derivatives of vitamin A, resulting in a photochemical response to visible or UV light. This work has lately centered on proteorhodopsin (pR), a solar-energy-transducing protein discovered in oceanic bacteria in 2000, and more recently in freshwater lakes around the globe. Solar energy transduction by pR throughout the biosphere is estimated to approach or exceed total worldwide human energy consumption (10 trillion Watts). A new emphasis in the lab is recently-invented process for carrying out solar-powered photochemical reduction of carbon dioxide, utilizing elemental bromine as an inexpensive photosensitizing agent.
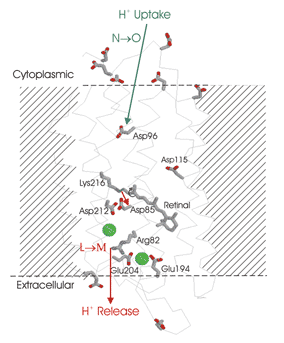
Spectroscopic studies of pR and related proteins. Our recent time-resolved FTIR spectroscopy of pR and the related protein bacteriorhodopsin indicates that the mechanism of proton pumping throughout this superfamily depends on a transient decrease in the proton affinity of a highly conserved arginine residue, leading to its release of a proton to the external side of the membrane. Ongoing site-directed mutagenesis and isotope-labeling studies, along with NMR experiments, are aimed at refining the details of this mechanism.
Use of a novel citrate-binding motif for membrane protein purification and crystallization. We have shown that pR has a citrate binding site involving several lysine residues in its first intramembrane loop. Upon binding low concentrations of citrate, pR selectively aggregates and is precipitated from detergent solution, without losing its native structure or functionality. This unusual property of pR is the basis for our recently-patented protein purification method, and for methods for crystallizing this protein. We are working to extend these results to the large family of G-protein-coupled receptors, by making chimeras of these receptors that incorporate the short citrate-binding motif of pR.
Small-molecule models for membrane protein active sites. The proton-releasing group of the microbial rhodopsins is modeled with a simple but novel synthetic molecule, consisting of an arginine side chain (guanidine) attached to a tyrosine side chain (phenol) via a long alkane linker. We are also investigating this class of compounds for possible pharmacological properties, since the arginine-tyrosine grouping is also highly conserved and mechanistically important to the visual rhodopsins and entire related superfamily of G-protein-coupled receptors.
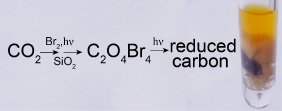
Bromine-sensitized solar photolysis of carbon dioxide. Elemental bromine and carbon dioxide were both first isolated in condensed phases in 1825. Despite this long history, no photochemical or thermal reactivity between Br2 and CO2 had previously been noted--until the Braiman lab's 2009 detection of a rich solar-powered photochemistry of ∼0.5% bromine in liquid CO2. The photoreaction rate to form the first easily-detectable metastable adduct depends supra-linearly on both photon and CO2 concentrations, and also appears to require a polar adsorbent substrate such as powdered silica or glass wool.
This Br2-sensitized photochemical reduction of CO2, which so far appear to store at least 1% of incoming solar energy in stable solid carbonaceous products, could play a role in sequestering CO2 from industrial sources, as well as in the solar-powered synthesis of new fuel feed-stocks. This could ultimately reduce our dependence on chlorophyll-containing living organisms, which up until now have carried out almost all of the solar-photochemical reduction of CO2 to useful fuels and synthetic building blocks. This bromine-sensitized inorganic photoreduction process, which does not require significant amounts of water, could permit more efficient utilization and storage of solar power falling on the Earth's deserts.
- M. Braiman. Bromine-sensitized solar photolysis of liquid CO2. US patents 9249508, published 2 Feburary 2016; and 9885111, published 6 February 2018.
- Experimental and computational modeling of H-bonded arginine-tyrosine groupings in aprotic environments. Banyikwa, A.T., Goos, A., Kiemle, D. J, Foulkes, M.A.C., Braiman, M.S. (2017) ACS Omega 2, 5641–5659. DOI:10.1021/acsomega.7b00282
- Anhydrous monoalkyl guanidines in aprotic and nonpolar solvents: models for deprotonated arginine side chains in membrane environments. Banyikwa, A.; Miller, S. E.; Gao, T.; Krebs, R. A.; Xiao, Y.; Carney, C.; Braiman, M. S. (2017) ACS Omega 2, 7239-7252. DOI: 10.1021/acsomega.7b00281
- R.W. Hendler, C. W. Meuse, M. S. Braiman, P. D. Smith, and J. W. Kakareka. Infrared and Visible Absolute and Difference Spectra of Bacteriorhodopsin Photocycle Intermediates”. Appl. Spectrosc. 65, 1029-1045 (2011).
- M.S. Braiman and Y. Xiao. "Step-scan time-resolved FTIR spectroscopy of biopolymers," in Vibrational Spectroscopy of Biological and Polymeric Materials, eds. V. Gregoriou and M.S. Braiman, CRC Press, Taylor and Francis group (ISBN 1-57444-539-1), pp. 353-418 (2007).
